




qRT-PCR 외에도 C12, C18, C22 유래의 Enhanced hiPS-HEP에서 면역형광염색을 진행하여 CYP1A2, CYP2C9, CYP3A4 발현을 확인하였다 (그림 2). 이를 통해 문맥 (Periportal)과 정맥혈 (perivecnous)
주위의 간세포에서 보이는 표현형과 유사한 CYP 효소의 발현을 연상할 수 있다 (Jungermann and Kietzmann 2000).

그림 2. Enhanced hiPS-HEP 세포의 면역형광염색
3종의 hiPSC (C12, C18, C22)로부터 분화된 enhanced hiPS-HEP 세포의 해동 6일 후 (Panel A), 해동 12일 후 (Panel B)에서 CYP1A2, 2C9, 3A4 발현을 염색으로 확인하였다.
다음으로 그림 3. Panel A의 CYP 효소의 활성을 LC/MS (liquid chromatography-mass spectrometry)로 분석하였다. Enhanced hiPS-HEP는 해동 20시간 후의 hphep와 유사한 CYP1A, CYP3A, CYP2C9 활성을 보였다
(그림 3. Panel B). CYP2B6, CYP2D6, CYP2C19의 활성도 확인 되었으나, hphep에 비해 낮은 수준으로 확인되었다. 중요한 것은 Enhanced hiPS-HEP에서 lot 별 CYP 활성 차이가 거의 미미한 수준으로 확인되었으며
(그림 3, Panel B, small error bar), 이는 분화 과정이 매우 안정적이라는 것을 의미한다. Richert et al. (2016) 에서 hphep을 2D로 배양했을 때 CYP 활성이 매우 빠르게 감소했던 반면, enhanced hiPS-HEP는
해동 4일 후부터 21일까지 CYP 활성이 안정적이었다 (그림 3, Panel C). 이를 종합해보면, Enhanced hiPS-HEP는 lot 별 variation이 낮고 배양 시간이 지속됨에 따라 CYP 활성이 안정적으로 유지되거나 증가함으로써,
연구자들에게 일관되고 안정적인 표현형의 세포를 제공할 수 있다.


그림 3. Enhanced hiPS-HEP 세포와 hphep 세포에서의 CYP 활성
(Panel A) CYP 활성 평가에 사용될 수 있는 CYP substrate와 각각의 대사 물질. 약물 대사에 미치는 영향, 즉 효소에 의해 대사되는 약물의 비율에 따라 순위가 매겨졌다 (Hewitt et al. 2007).
(Panel B) 3종의 hiPSC (C12, C18, C22)로부터 분화된 enhanced hiPS-HEP (해동 4일, 12일, 19일 후)와 hphep (해동 20시간 후)에서 CYP 활성을 분석하였다.
(Panel C) 3종의 hiPSC (C12, C18, C22)로부터 분화된 enhanced hiPS-HEP (해동 4일, 8일, 12일, 15일, 19일, 21일 후)에서 CYP 활성을 분석하였으며, 6종의 CYP 효소에서 모두 21일 동안 안정적인 발현을 보였다.
흥미롭게도, 서로 다른 hiPSC에서 분화된 enhanced-hiPS-HEP는 각기 다른 CYP 활성을 보이며, 이는 개체 간 CYP 특성의 변이를 반영하고 있다 (그림 3, Panel B). 예를 들면, C12, C22 유래보다 C18 유래의 enhanced hiPS-HEP에서
더 높은 CYP3A 활성을 보였다. CYP3A 동종 효소인 CYP3A4, CYP3A5, CYP3A7은 활성 분석으로 구분할 수 없기 때문에, qRT-PCR 분석 (그림 1)을 이용해 동종 효소와 세포주 간의 발현 변화를 확인했다.
그림 1에서 C18 유래의 enhanced hiPS-HEP는 C12, C22 유래의 세포보다 더 높은 CYP3A5 발현을 보이는 반면, CYP3A4는 유사한 발현을 보였다.
Effective expression and activity of phase II enzymes over an extended culture time
Phase II 효소 또한 약물 대사 과정에 관여한다. SULT (sulfotransferases)와 UGT (uridine diphosphate glucuronosyltransferase)와 같은 phase II 효소는 대부분의 화합물의 용해도를 증가시켜 신장으로 배설할 수 있게 한다. Enhanced hiPS-HEP에서
Phase II 효소 활성과 발현을 확인하고자 여러 방법을 이용하였다. 먼저, qRT-PCR 분석을 통해, 해동 4일, 20일 후의 enhanced hiPS-HEP에서 UGT1A1, UGT2B7 mRNA 발현이 안정적으로 유지되거나
약간 증가하는 것을 확인하였다 (그림 4, Panel A). 이 mRNA 발현은 hphep와 유사함을 확인하였다 (그림 4, Panel B). SULT와 UGT 효소 활성은 LC/MS로 분석하였으며 (그림 4, Panel C),
유래한 hiPSC에 관계없이 hphep과 유사한 높은 효소 활성을 보였다. Enhanced hiPS-HEP는 해동 4일 후와 19일 사이에 SULT, UGT 를 안정적으로 발현하거나, 발현이 약간 증가되었다.
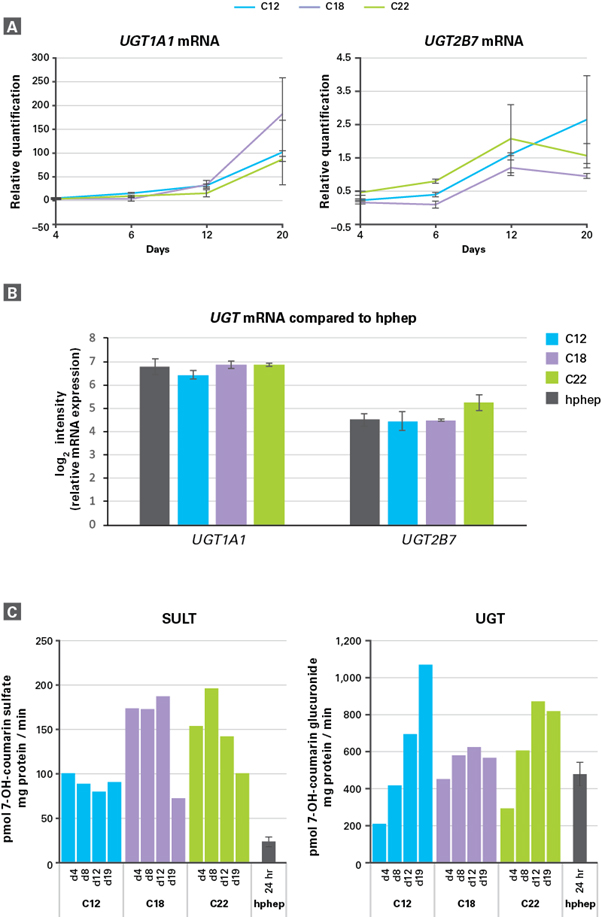
그림 4. Enhanced hiPS-HEP에서 보이는 phase II 효소의 기능 발현
(Panel A) 3종의 hiPSC (C12, C18, C22)로부터 분화된 enhanced hiPS-HEP (해동 4일, 20일 후)에서 UGT1A1, UGT2B7 발현 확인
(Panel B) 3종의 hiPSC (C12, C18, C22)로부터 분화된 enhanced hiPS-HEP (해동 13일 후)와 hphep (해동 1일 후)에서의 UGT1A1, UGT2B7 효소의 mRNA 발현을 정량 분석하여 비교하였다.
(Panel C) Enhanced hiPS-HEP를 LC/MC로 분석했을 때 배양 시간에 따라 높은 SULT 활성을 보였으며, 이를 통해 SULT 대사산물인 7-OH-coumarin sulfate와 UGT 대사산물인 7-OH-coumarin glucuronide가 축적됨을 확인할 수 있다.
Hepatic uptake and efflux transporter expression
Phase I, II 효소와 함께, enhanced hiPS-HEP의 uptake transporter (NTCP, OCT1, OATP1B1, OATP1B3)와 efflux transporters (MRP2, MDR1, BSEP)
발현을 hphep와 비교하였다. Enhanced hiPS-HEP에서 qRT-PCR 분석을 진행했을 때, phase I, II 효소와 마찬가지로 transporter 발현이 안정적이거나 약간 증가하는 것을 확인하였다 (그림 5, Panel A-B).
그림 5, Panel C에서 hphep와 비교했을 때, enhanced hiPS-HEP가 대부분의 efflux transporter와 uptake transporter NTCP가 유사한 수준으로 발현한 반면, OATP1B1, OATP1B3, OCT1는 낮게 발현하였다.
C18 유래의 enhanced hiPS-HEP에서는 OATP1B3 발현이 확인되지 않았다.

그림 5. Enhanced hiPS-HEP와 hphep에서의 transporter mRNA 발현
(Panel A-B) 3종의 hiPSC (C12, C18, C22)로부터 분화된 enhanced hiPS-HEP (해동 4일, 20일 후)에서 uptake transporter, efflux transporter 유전자에 대한 mRNA 발현 수준을 확인하였다.
(Panel C) 3종의 hiPSC (C12, C18, C22)로부터 분화된 enhanced hiPS-HEP (해동 13일 후)와 hphep (해동 1일 후)에서 transporter mRNA 발현을 비교하였다.
*MDR1= multidrug resistance protein 1; BSEP = bile salt export pump; MRP2 = multidrug resistance-associated protein 2; NTCP = Na+-taurocholate co-transporting polypeptide; OCT1 = organic cation transporter 1; OATP1B1/1B3 = organic anion transporting polypeptide 1B1/1B3.
Proof-of-concept chronic toxicity study
Enhanced hiPS-HEP에서 약물 대사에 중요한 역할을 하는 효소들의 발현과 활성을 확인하였다. 다음은 이 세포들이 잘 알려진 간 독성 약물을 이용해 만성 독성 연구에 적절한 반응이 있는지를 확인하였다. 이에 주로 사용되는 간 세포 모델인 3D spheroid 형태의 hphep와 간암세포주인 HepaRG에서도 분석을 진행하였다.
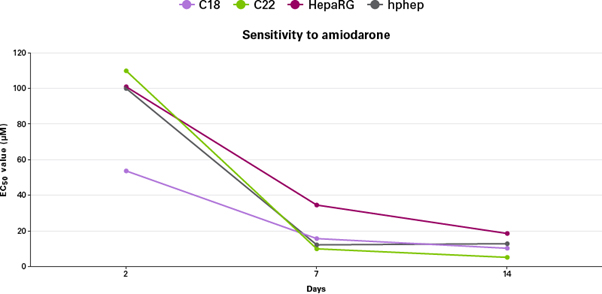
그림 6은 각 hphep (Panel A), HepaRG 세포주 (Panel B), C18 유래의 enhanced hiPS-HEP (Panel C), C22 유래의 enhanced hiPS-HEP (Panel D)에 aflatoxin 처리 후의 세포 생존능을 확인한 결과다. Enhanced hiPS-HEP의 경우 해동 4일 후에 aflatoxin을 처리하였으며, 처리 후 2일, 7일, 14일 후에 생존능을 확인하였다. 용량반응곡선 (Dose-response curve)은 세포 생존율에 따라 작성되었다. 표 1에서는 4종의 물질을 처리했을 때, 처리 7일차 혹은 14일 차에 EC50 값의 감소를 보여주었으며, 그림 6의 용량반응곡선이 왼쪽으로 이동한 것과 같은 결과를 얻었다. 이는 예상했던 바와 같이, 실험에 사용된 모든 세포군에서 처리 물질을 장기간 노출함에 따라 감도가 증가했음을 의미한다 (그림 7).

그림 6. Enhanced hiPS-HEP에서 화합물 처리시간에 따라 감도가 증가함을 나타내는 용량반응곡선
약물 패널에 사용되는 대표적인 물질인 aflatoxin을 세 농도로 각 세포군에 처리하였다. 용량반응곡선은 각 hphep (Panel A), HepaRG 세포주 (Panel B), C18 유래의 enhanced hiPS-HEP (Panel C),
C22 유래의 enhanced hiPS-HEP (Panel D)로 테스트하였다.
Cell type |
Timepoint (Days) |
Amiodarone |
Aflatoxin |
Troglitazone |
Chlorpromazine |
hphep cells |
2 |
100 |
0.62 |
32.4 |
15.5 |
7 |
12.3 |
0.09 |
4.5 |
8.1 |
|
14 |
12.8 |
0.02 |
1.4 |
5.2 |
|
HepaRG cells |
2 |
101 |
1.3 |
- |
67 |
7 |
34.7 |
0.3 |
36.5 |
32.4 |
|
14 |
18.5 |
0.1 |
34.6 |
34.4 |
|
Cellartis® enhanced hiPS-HEP cells |
2 |
53.8 |
89 |
221.5 |
213.6 |
7 |
15.9 |
0.6 |
132.5 |
20 |
|
14 |
10.5 |
0.1 |
114.5 |
7.4 |
|
Cellartis® enhanced hiPS-HEP cells |
2 |
110 |
401.1 |
262 |
47.8 |
7 |
10.1 |
6.4 |
153.4 |
14.8 |
|
14 |
5.2 |
6.9 |
168.7 |
15 |
Code |
제품명 |
용량 |
Y10133 |
1 Kit |
|
Y10134 |
1 Kit |
|
Y10135 |
1 Kit |